Isotope Practice Worksheet Answers
Isotope Practice Worksheet Answers - Use isotope notation to determine: Fill in the following table. The numbers 12, 13, and 14 refer to the mass number. Web elements & isotopes quiz. This quiz aligns with the following ngss standard (s): Element name/symbol, atomic number, number of electrons, number of neutrons, number of protons, mass number, atomic number, atomic mass.
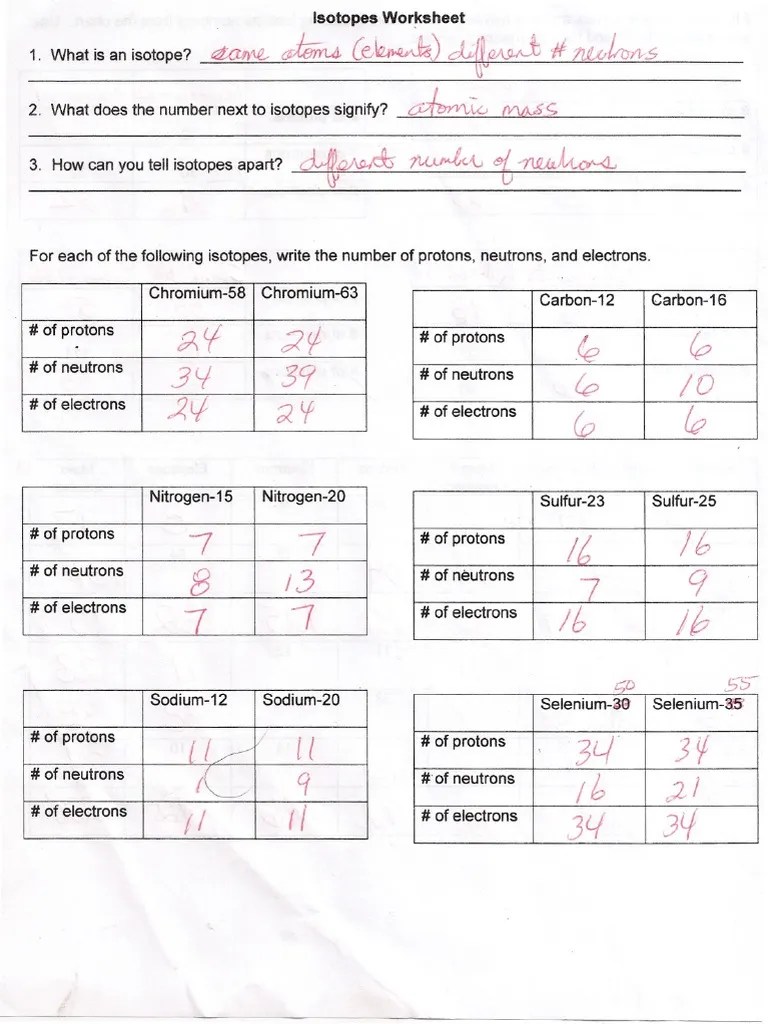
Chromium58 chromium63 # of protons 24 24 # of neutrons 34 39 Ion and charged isotope practice. Use the periodic table to identify and count subatomic particles within the atom. Select your preferences below and click 'start' to. 5 protons, 6 neutrons, 5 electrons.
Web isotope practice worksheet 1. How can you tell isotopes apart? Students shared 5612 documents in this course. Web elements & isotopes quiz. Web isotope and ions practice worksheet name:
19 protons, 22 neutrons, 19 electrons. What does the number next to isotopes signify? The numbers 12, 13, and 14 refer to the mass number. Web isotope and ions practice worksheet name: Web isotope practice worksheet name:
How can you tell isotopes apart? You would get a different element Web ion and charged isotope practice worksheet | live worksheets. Click the card to flip 👆. Isotopic abundance = 90.92% (isotope 2) mass = 21 amu;
Use isotope notation to determine: Web isotopes practice set 1. How many protons and neutrons are in the first. What does the number next to isotopes signify? What does the number next to isotopes signify?
The numbers 12, 13, and 14 refer to the mass number. 5 protons, 6 neutrons, 5 electrons. Click the card to flip 👆. Web isotope practice worksheet name: For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units).
How can you tell isotopes apart? (isotope 1) mass = 20 amu; The numbers 12, 13, and 14 refer to the mass number. This document has been uploaded by a student, just like you, who decided to remain anonymous. Web isotopes worksheet answer key.
Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses: Web isotopes worksheet answer key. Here are three isotopes of an element: What does the number next to isotopes signify? Web calculate the average atomic mass for the following element given information about the relevant isotopes:
Protons and neutrons are in the nucleus. Web elements & isotopes quiz. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. What does the number next to isotopes signify? A chemical element's isotope is a distinct form with a different number of neutrons in its nucleus.
Web calculate the average atomic mass for the following element given information about the relevant isotopes: The numbers 12, 13, and 14 refer to the ________________________ d. What does the number next to isotopes signify? Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; The numbers 12, 13, and 14 refer to the mass number.
(b) atomic number 43, mass number 99, charge of 7+. Web isotopes practice set 1. Web iodine is 80% 127i, 17% 126i, and 3% 128i. Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses: Web ion and charged isotope practice worksheet | live worksheets.
How can you tell isotopes apart? (a) atomic number 9, mass number 18, charge of 1−. Students shared 5612 documents in this course. The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. 19 protons, 22 neutrons, 19 electrons.
Isotope Practice Worksheet Answers - Web iodine is 80% 127i, 17% 126i, and 3% 128i. (a) atomic number 9, mass number 18, charge of 1−. For each of the following isotopes, write the number of protons, neutrons, and electrons. Web ðï ࡱ á> þÿ s u. I can use the atomic number of an element to identify protons and electrons for any element. Select your preferences below and click 'start' to. 19 protons, 22 neutrons, 19 electrons. Hydrogen is 99% 1h, 0.8% 2h, and 0.2% 3h. How can you tell isotopes apart? (isotope 1) mass = 20 amu;
Ion and charged isotope practice. (b) atomic number 43, mass number 99, charge of 7+. Click the card to flip 👆. I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element. Fill in the following table.
This online quiz is intended to give you extra practice in writing names and notation for hundreds of elements and isotopes. Isotopic abundance = 90.92% (isotope 2) mass = 21 amu; What does the number next to isotopes signify? The natural abundance for boron isotopes is 19.9% 10b and 80.1% 11b.
Calculate The Average Atomic Mass Of Chlorine If Its Isotopes And % Abundances Are As Follows.
Web isotopes practice set oe aa — 1. (isotope 1) mass = 20 amu; Select your preferences below and click 'start' to. This document has been uploaded by a student, just like you, who decided to remain anonymous.
The Atomic Mass For Each Element Appearing On The Periodic Table Represents The Weighted Average Of Masses For Each Individual Isotope Of An Element.
Web isotopes practice set 1. The natural abundance for boron isotopes is 19.9% 10b and 80.1% 11b. Web ion and charged isotope practice worksheet | live worksheets. Web elements & isotopes quiz.
How Many Protons And Neutrons Are In The First.
How can you tell isotopes apart? Calculate its average atomic mass. How many protons and neutrons are in the first isotope? How can you tell isotopes apart?
Any Of Two Or More Versions Of A Chemical Element, Having The Same Number Of Protons In The Nucleus, Or The Same Atomic Number, But Having Different Numbers Of Neutrons In The Nucleus, Or Different Atomic Masses.
This quiz aligns with the following ngss standard (s): Element name/symbol, atomic number, number of electrons, number of neutrons, number of protons, mass number, atomic number, atomic mass. 5 protons, 6 neutrons, 5 electrons. What would happen if the number of protons were to change in an atom?