Isotope Notation Worksheet Answers
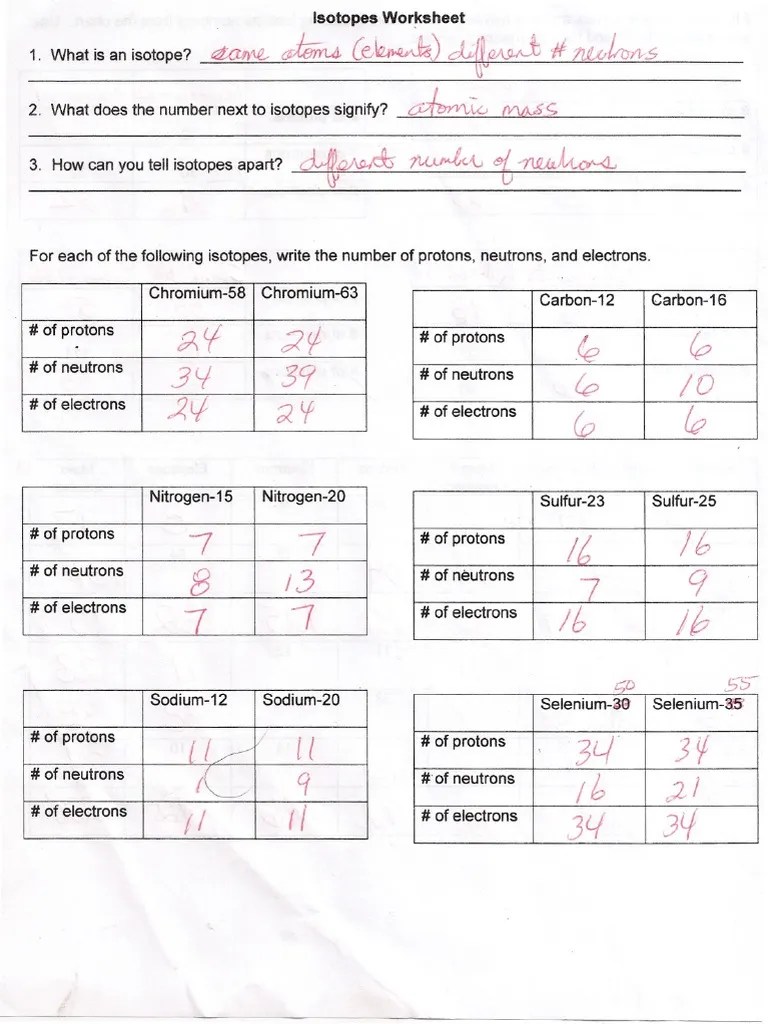
Isotope Notation Worksheet Answers - How do the isotopes of an element differ? Use isotope notation to determine: Identify which among the isotope symbols below is incorrect. Of protons but different no. Here are three isotopes of an element: Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres.
I,iu, atoul t1 3o lb lrl lll nthlloil pnllojjr. Web fill in the blanks in the following worksheet. I can write the hyphen notation for any isotope given protons, neutrons, and electrons and use the hyphen notation to find protons, neutrons, and electrons for an atom. Web ðï ࡱ á> þÿ s u. Atomic number, and ions have charge!
____carbon_____ the number 6 refers to the _____atomic number _____ What is the isotope notation of the element that has an atomic number of 24 and a mass number of 52? Web isotope and ions practice worksheet name: Web element classification (e.g., metal, nonmetal, metalloid) element state (e.g., solid, liquid, gas) isotope notation. Included in the chemistry instructor resources subscription.
Ions, ion notation, electrons, anions, cations, isotopes, isotope notation, neutrons, atomic mass. The numbers 12, 13, and 14 refer to the mass number. Isotopes are atoms of the same element with different atomic masses. Web element classification (e.g., metal, nonmetal, metalloid) element state (e.g., solid, liquid, gas) isotope notation. Name tsl**/ atom or ion?
How many protons and neutrons are in the first isotope? Here are three isotopes of an element: Web ðï ࡱ á> þÿ s u. Isotopes, isotope notation, neutrons, atomic mass. Web this worksheet will give students some visual examples of how isotopes are different, and allow them to practice writing isotope notation.
612 c 613 c 614 c a. (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. Here are three isotopes of an element: I,iu, atoul t1 3o lb lrl lll nthlloil pnllojjr. Web i can write the hyphen notation for any isotope given protons, neutrons, and electrons and use the hyphen notation to find.
Here are three isotopes of an element: Included in the chemistry instructor resources subscription. Web this worksheet will give students some visual examples of how isotopes are different, and allow them to practice writing isotope notation. How are they the same? Use isotope notation to determine:
Web ðï ࡱ á> þÿ s u. When symbols are used to. (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. Please keep in mind that the isotope represented by each space may not be the most common isotope or the one closest in atomic mass to the value on the periodic table. How.
Ffie atom /, 2q /& /& /i t'le{t si [1.o,. Isotopes are atoms of the same element with different atomic masses. The numbers 12, 13, and 14 refer to the mass number. The number 6 refers to the _____ c. Atomic number, and ions have charge!
Of protons but different no. Included in the chemistry instructor resources subscription. Pagel of 2 40 15 32 63 125 '33 j. When symbols are used to. Web isotope and ions practice worksheet name:
Use isotope notation to determine: Students shared 5678 documents in this course. ____carbon_____ the number 6 refers to the _____atomic number _____ Students need a periodic table and need to know what the mass number and the atomic number of the elements mean. Ions, ion notation, electrons, anions, cations, isotopes, isotope notation, neutrons, atomic mass.
Element name/symbol, atomic number, number. Meave60 · 1 · may 26 2015. Students need a periodic table and need to know what the mass number and the atomic number of the elements mean. Students shared 5678 documents in this course. Isotopes, isotope notation, neutrons, atomic mass.
Web chemistry worksheet isotope notation name: I,iu, atoul t1 3o lb lrl lll nthlloil pnllojjr. 612 c 613 c 614 c a. A worksheet of practice ion and isotope notation questions. E) 48− 22 = 26.
Isotope Notation Worksheet Answers - What part of the atom contains practically all its mass? The number of determines the name of the atom. A worksheet of practice ion and isotope notation questions. Meave60 · 1 · may 26 2015. Here are three isotopes of an element: The numbers 12, 13, and 14 refer to the mass number. Web element classification (e.g., metal, nonmetal, metalloid) element state (e.g., solid, liquid, gas) isotope notation. Of neutrons at its nucleus. Students need a periodic table and need to know what the mass number and the atomic number of the elements mean. How are they the same?
I can write the hyphen notation for any isotope given protons, neutrons, and electrons and use the hyphen notation to find protons, neutrons, and electrons for an atom. The numbers 12, 13, and 14 refer to the mass number. Web this worksheet will give students some visual examples of how isotopes are different, and allow them to practice writing isotope notation. Web i can write the hyphen notation for any isotope given protons, neutrons, and electrons and use the hyphen notation to find protons, neutrons, and electrons for an atom. The atom with 79 protons and 117 neutrons.
Web element classification (e.g., metal, nonmetal, metalloid) element state (e.g., solid, liquid, gas) isotope notation. 612 c 613 c 614 c a. (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. Web i can write the hyphen notation for any isotope given protons, neutrons, and electrons and use the hyphen notation to find protons, neutrons, and electrons for an atom.
Isotopes Are Basically Atoms Which Have The Same No.
The number of protons in an atom is known as the of that plus the atom. Web chemistry worksheet isotope notation name: Which element contains the largest number of neutrons per atom? (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe.
Ions, Ion Notation, Electrons, Anions, Cations, Isotopes, Isotope Notation, Neutrons, Atomic Mass.
The atom with 79 protons and 117 neutrons. The number of determines the name of the atom. 612 c 613 c 614 c a. It could be good for a chemistry course or general science course that covers the periodic table.
Included In The Chemistry Instructor Resources Subscription.
Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. Atomic number, and ions have charge! The numbers 12, 13, and 14 refer to the mass number. Ffie atom /, 2q /& /& /i t'le{t si [1.o,.
E) 48− 22 = 26.
Web this worksheet has 20 problems on writing elements in isotope notation. What is the isotope notation of the element that has an atomic number of 24 and a mass number of 52? Name tsl**/ atom or ion? Isotopes are atoms of the same element with different atomic masses.